Highlight
Genetics and Brain Imaging Together Shed Light on Smoking Cessation
Achievement/Results
Researchers at the University of Pittsburgh’s Center for the Neural Basis of Cognition have combined genetic testing with functional brain imaging to provide a new view of the difficulties facing smokers trying to give up their habit. One of the factors influencing ability to overcome addictive behavior is how one’s brain processes reward signals. Dopamine is a neurotransmitter associated with reward. Variations in the dopamine receptor genes DRD4 and Taq1A have been suggested to affect reward responses in smokers during periods of nicotine deprivation.
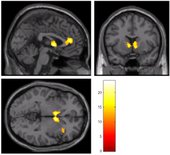
To test this hypothesis, psychology doctoral candidate Maggie Sweitzer and her advisor, Professor Eric Donny, studied a group of 44 daily smokers who agreed to participate in a smoking cessation experiment. First they conducted a genetic analysis, looking for variations in the DRD4 and Taq1A genes. Next they had subjects perform a task in an fMRI (functional magnetic resonance imaging) scanner where they could earn both smoking and monetary rewards. Each subject experienced two conditions: a satiated condition where they had been smoking as usual prior to the experiment, and an abstinent condition in which they had refrained from smoking for the past 24 hours. A comparison of responses to the two rewards across the two conditions revealed several important findings. Abstinence was associated with a significant increase in response to smoking rewards and a decreased response to monetary rewards in three brain areas: bilateral caudate nucleus, medial prefrontal cortex, and right insula, as shown in the accompanying figure. In other words, nicotine withdrawal caused the smokers’ brains to value smoking more highly, and money less highly. These results provide, for the first time, empirical support for theories suggesting an abstinence-induced bias in reward processing. In the next phase of the study, subjects participated in a three-week smoking cessation program using an internet-based contingency management procedure in which they received a monetary incentive for each smoke-free day. Biochemical testing was used to verify abstinence. Thus, smokers faced a daily choice between the immediate gratification of a cigarette vs. the promised monetary reward for abstaining. Individuals whose brain scans had shown a greater drop in response to monetary reward in the caudate nucleus were found to be more likely to lapse during the cessation program, when abstinence was reinforced with money. This could indicate that decreased responsiveness to non-drug rewards during abstinence may be a mechanism underlying vulnerability to continued smoking.
Sweitzer and Donny then examined whether genotype predicted the change in neural response to each type of reward as a function of abstinence. Carriers of the A1 allele of the Taq1A gene exhibited a significantly smaller decrease in bilateral caudate and anterior cingulate activation in anticipation of a puff reward vs. a monetary reward during abstinence than non-carriers. This attenuated difference score was found to be driven by greater activation in anticipation of puff reward during non-abstinence. This could indicate that carriers of the A1 allele experience a dimished satiation effect after smoking, potentially increasing their vulnerability to persistent smoking behavior. In contrast, no differences were found for carriers of the 7R allele of the DRD4 gene. Results of the fMRI portion of the study were reported at the Society for Neuroscience meeting in October 2012.
Sweitzer’s research, combining behavioral, genotyping, and brain imaging methodologies, was supported in part by a National Science Foundation IGERT (Integrative Graduate Education and Research Training) fellowship. The IGERT program at the Center for the Neural Basis of Cognition provides cross-disciplinary graduate training to create the next generation of brain scientists who are able to study cognition at multiple levels, from biochemistry to behavior. Sweitzer writes: “I believe the IGERT fellowship has been an integral part of my overall graduate training, and I am excited to continue to build on the foundational skills in genetics and neuroimaging that I learned through this training.” She will be pursuing postdoctoral work at Duke University, where she will continue her research examining genetic and neuroimaging predicators of smoking behavior.
Address Goals
A better understanding of the mechanisms underlying drug and alcohol addiction is necessary for the development of more effective treatments. Genetic factors may help explain why some people are more successful than others at shedding addictive behaviors. Sweitzer’s research advances our understanding of how the brain shifts its valuation of reward as a result of nicotine deprivation, and how genetics influences that shift. The multi-disciplinary character of Sweitzer’s work required extensive training which would not have been feasible without the support she received through the IGERT program. She will graduate in August 2013 and begin postdoctoral training, the next step toward what promises to be a productive scientific career.