Highlight
Application of Fractional Factorial Design to the Biosynthetic Production of Hydrogen
Achievement/Results
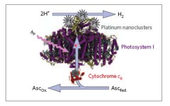
The interdisciplinary research team of Dr. Paul Frymier (Chemical Engineering), Dr. Barry Bruce (Biochemistry), and Dr. Rupy Sawhney (Industrial Engineering) developed a model to optimize the deposition of nanometer-scale clusters of platinum on Photosytem I (PSI) protein complexes from cyanobacteria used in the production of hydrogen as a potential fuel source. The formation of appropriately sized platinum nanoclusters on the end of cyanobacterial photosystems is a critical step in the self-organization of these hydrogen evolving nanoclusters. So far however, a heuristic approach has been used to select the experimental conditions for forming the nanoparticles. This led to considerable process variability due to the number of variables that must be fixed for a particular experiment, including temperature, protein and platinum concentrations, and the amount and wavelength of light used to photooxidize the photosystems.
Drs. Frymier and Bruce had already been collaborating on the generation of hydrogen using the photosystems from cyanobacteria (see Fig. 1), as advisors for a research team that included graduate student NSF IGERT Trainee Rosemary Le and Associate Ifeyinwa Iwuchukwu. When Ernest Iwuchukwu joined the lab of Dr. Sawhney, dinner conversations between Ifeyinwa and Ernest lead to a discussion of how to fully explore the combination of possible variables without running hundreds of experiments that each take days to run. These conversations led Dr. Sawhney to join the team and bring expertise in the area of process optimization. The result was the use of fractional factorial design to build a multivariate model for the process. The team was interested to know which variables were the most important and also if interactions between the variables were as important as the individual variables themselves. Second year Trainee Le got involved by learning to use the equipment and developing the schedule for the experiment, which lasted several weeks and required the coordination of the schedules of several people, including undergraduate students working on the project.
The team was surprised to find that the effect of variable that were thought to be critical were much less important than others thought to be not important. The platinum concentration had the largest effect on the amount of hydrogen generated while the amount of light used and the temperature had a lesser effect. The interaction of the temperature and the platinum concentration was determined to be important, but not the wavelength and amount of light, which was contrary to conventional wisdom (see Fig. 2). When the wavelength of the light used to form the platinum nanoclusters was studied, it was found that the use of red light alone led to higher levels of hydrogen production than the equivalent amount of red light, with additional shorter wavelengths of light. The mechanism for the sub-optimum result when lower wavelengths of light are used is not known, but it is thought to be a result of possible photodamage from exposure to blue light.
In these experiments, the integrated yield of the first three peaks after the start of the hydrogen evolution phase was chosen as the dependent variable. Using JMP, statistical data analysis software, a model equation was developed to predict an optimum for hydrogen evolved, after platinization, under the optimal conditions predicted. Visual observation of the surface and contour plots, above, also indicated that an optimal yield would be maximized at a light intensity of 236 E m-2 s-1, platinum salt concentration of 636 M and temperature of 30.10C. The predicted optimum was 8.17 µmol H2 h-1 mg chl-1. To validate the model, another experiment was performed with observed optimal conditions during platinization. The hydrogen evolution portion of the experiment was performed at the same conditions as for all of the previous experiments, a light intensity of 280 uE m-2 s-1, 10x molar excess of cytochrome and a temperature of 350 deg C . The hydrogen yield for the validation experiment was 8.02 µmol H2 h-1 mg chl-1, a value higher than observed in previous experiments used to derive the model. The yields from the central composite experiments are summarized in Fig. 3 with the optimized case highlighted in green.
Address Goals
This research project applies fractional factorial design to the optimization of a process for generating a promising transportation fuel (hydrogen) in a potentially sustainable fashion. Through the consideration of multiple process variables and their interactions, the process optimum was found much more quickly and efficiently than using a complete factorial design or heuristic approach. The use of this methodology is particularly important for problems involving biological systems, since the materials often have to be generated for each experiment through expensive and time-consuming laboratory work. Furthermore, the project was initiated and fostered by the interdisciplinary nature of the IGERT program; this project involved students from industrial and chemical engineering as well as biochemistry. The students from each discipline participated in some way in every part of the project and so gained an appreciation and basic knowledge of the utility of application of both biological and chemical science and engineering as well as the application of procedures for conducting experiments in the most efficient and cost-effective manner.