Highlight
The role of mineral nanoparticles as transporters of toxic trace metals: A case study from the Clark Fork River Superfund Complex
Achievement/Results
EIGER IGERT trainee Kelly Plathe, under the leadership of EIGER PI Michael Hochella, has proven that mineral nanometer-sized particles are responsible for transporting toxic heavy metals hundreds of kilometers downstream in one of the largest contaminated sites in the United State (the Clark Fork River Superfund Site, Montana). This discovery is seminal because mineral nanoparticles in complex natural systems are particularly difficult to extract and characterize, and no study has ever accomplished this task in as much detail, and with as much precision, as this study. This discovery adds another vital component in our practical understanding of how toxins are transported in the environment over great distances, and it can be used in the future to assess the bioavailability of the most toxic metals like lead and arsenic.
Nanoparticles, which have at least one dimension less than a few tens of nanometers, can interact with and/or affect contaminants differently (and sometimes dramatically differently) than similar particles that are just larger in size. One of the main reasons nanoparticles are so reactive is that as the size of a particle decreases, the ratio of its surface area to volume increases dramatically, thereby increasing the amount of surface available for reactions. However, while many property changes are a result of this increased ratio, some are due to variations in surface properties and chemistry that only occur when the particle exists in the nano-size range. Because of their small size, nanoparticles are also capable of staying suspended in rivers and ground water for long periods of time, or indefinitely, under conditions not conducive to extensive, dense aggregation due to, e.g. pH, ionic strength, and organic molecule attachment, or attachment to other, larger particles.
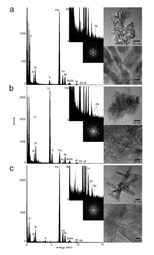
In this study, sediment samples were taken from the Clark Fork River in western Montana, USA. Over the past 150 years, base metal mining released significant amounts of metals (most notably lead, arsenic, zinc and copper) into the Clark Fork river/floodplain system at levels toxic to the environment, resulting in the largest Superfund Complex in the United States. In order to take a closer look at the nano-mineral – heavy metal relationships in this system, it was decided to employ a density fractionation technique, using sodium polytungstate, to separate the higher density, more metal-rich oxide mineral particles from the lower density clay particles. This separation was coupled with a nanoparticle extraction method to separate the dense nanoparticulate fraction from the bulk sediment. All samples were analyzed using asymmetrical flow field-flow fractionation (aFlFFF) coupled to multi-angle laser light scattering (MALLS) and high resolution – inductively coupled plasma mass spectroscopy (HR-ICPMS), as well as analytical transmission electron microscopy (aTEM). aFlFFF analysis revealed a size distribution in the nano-range and that the elution profiles of the trace metals matched most closely to that for Fe and Ti. aTEM confirmed these results as the majority of the particles analyzed were Fe and Ti oxides and were associated with one or more of the trace metals of interest.
Using these techniques and protocols, we discovered that the main mineral phases hosting metals are nanoparticles of the minerals goethite, ferrihydrite and brookite (these are iron and titanium oxide minerals). This study demonstrates that these mineral nanoparticles are playing a key role in determining the transport, bioavailability, and toxicity of contaminant trace metals within the Clark Fork Superfund Site and beyond, further downstream. This river system is extremely multivariate and complex, as any natural system is, but we have been able to uncover the workings of one portion of its smallest component. This is important as industry continues to make significant advances in nanotechnology, accompanied by the intentional and unintentional release of manufactured nanoparticles into the environment. The more we understand now about how natural nanoparticles are affecting their surrounding, the more we will be able to predict what may be happening with man-made nanomaterials in the environment.
Address Goals
Discovery is the NSF strategic goal that is primary in this Highlight because the importance and opportunities surrounding this research are great. Contamination of soils and sediments with heavy metal(loid)s is a worldwide problem stemming from many anthropogenic activities, such as ore mining, metal purification (e.g. flotation and smelting of ores or preparation of nuclear fuels), and burning fossil fuels. Elevated concentrations of metal(loid)s can also be produced naturally (e.g. arsenic contamination of groundwater in the Ganges Delta). Whatever the source, elevated concentrations of metal(loid)s are known to have adverse effects on humans, fauna and flora.
Remediation techniques must attempt to remove or isolate/immobilize them from whatever geosystem they are held in, and all geosystems are invariably complex. Before remediation can occur, one must have information regarding how the contaminant of interest is contained within and interacting with its environment. If the contaminants are found to be associated with nanoparticles, as in this case, the opportunities are particularly great, as nanoparticle behavior is complex and unusual. The properties of nanoparticles are often not equivalent to larger particles of the same material, making nanoscience and technology a field that is incredibly exciting and vibrant as more is understood about the behavior of nanomaterials.
Learning is the NSF strategic goal that is secondary in this Highlight because we are helping to build a broadly trained workforce by educating trainees in highly interdisciplinary ways, while at the same time getting them to appreciate specific science and engineering disciplines. In this case, in an international, interdisciplinary project that involved EIGER trainee Kelly Plathe, EIGER PI Michael Hochella, Prof. Frank von der Kammer at the University of Vienna, Austria, and Prof. Martin Hassellov at the University of Goteborg, Sweden, a scientific breakthrough was achieved that is a direct result of what this particular IGERT program makes possible. The research involves the movement of toxic heavy metals in the environment, in this case over hundreds of kilometers in one of the largest contaminated sites in the United State (the Clark Fork River Superfund Site, Montana). This international research team has now written multiple, but still basic, review articles for various journals and magazines that can be understood by any educated citizen. News of our discoveries have also been described in publications accessible to the general public, as well as mass media such as on National Public Radio.