Highlight
Production and Stabilization of Pharmaceutical Nanoparticles using an Environmentally Safe Emulsion Template
Achievement/Results
1. Production of Pharmaceutical Nanoparticles using an Environmentally Safe Emulsion Template
In the past decade, the poor water-solubility of many drugs has become a focal point of the pharmaceutical industry. In addition, it has also driven the innovation of several novel techniques to administer safe and effective drug levels. For instance, pharmaceutical actives can be created in the form of nanoparticles. Nanoparticles have been proven to achieve a faster solubility rate, and thus, a higher bioavailability of the drug. In addition, nanoparticles exhibit many other unique advantages for drug delivery including: passive targeting, ease of suspension, variable optical properties, and the ability to be functionalized for various applications.
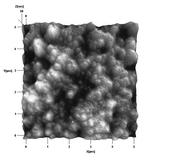
A novel process for the production of pharmaceutical nanoparticles has since been developed by an IGERT research team consisting of Dr. M. S. Tomassone, Dr. P. Takhistov and associated students. This new process, entitled the Emulsion-Diffusion process actually creates nanoscale drug particles in suspension without encapsulation in a solid lipid as used in traditional emulsion techniques. In this process, emulsion droplets act as the site for the nucleation and subsequent growth of pharmaceutical nanocrystals. Specifically, the active drug is dissolved in the dispersed oil phase of an emulsion that is partially miscible and in equilibrium with the continuous water phase. The emulsion is then homogenized, adding large amounts of shear and cavitation to the emulsion to produce a nanoemulsion containing droplets only several hundred nanometers in diameter. The nanoemulsion is then introduced into a well mixed system of additional anti-solvent which quickly dissolves the partially miscible oil phase. The dissolution of the oil-phase droplet results in the supersaturation of the drug inside the droplet allowing crystallization to occur. The small crystals are then subsequently stabilized using a relatively high concentration of emulsifier already present in the system. The resulting product is a well dispersed suspension of 100nm diameter particles that remains stable for several days. Figure 1 is an Atomic Force Microscopy image of the created nanoparticles using a Butyl Lactate oil phase and Griseofulvin as the active drug.
The emulsion-diffusion process has additional advantages over traditional techniques. Incidentally, the entire developed process can be conducted using only ingredients commonly used as food additives. The oil phase (dispersed) can be constructed of Triacetin or n-Butyl Lactate, both common solvents used in the food and drug industry. This effectively eliminates the need for harsh solvents commonly used in many bottom-up synthesis techniques. In addition, the emulsions can be stabilized using a natural stabilizer: Soy Lecithin. This commonly used emulsifier in the food industry replaces the high concentrations of non-ionic and ionic surfactants that are usually required in other nanoparticle creation techniques. Finally, this process is done using a high pressure homogenizer, an easily scalable technique. Since this process only uses 2-3 homogenization passes, several homogenizers can be placed in series for a continuous, one-step operation.
2. Stabilization of Drug Particles studied using Molecular Dynamics Simulations
When creating nano- and micron- sized particles of pharmaceuticals, stability has become a major focal point for modern research. In addition, it has become increasingly important to use surfactants that are widely accepted by the FDA. Unfortunately, few models exist for the selection of such surfactants, specifically for work with pharmaceuticals. Most industries rely on empirical data or classic trial-and-error approaches for determining the optimal surfactants for their particular system. However, new computational techniques have been developed for the actual understanding of surfactant behavior. Dr. M. S. Tomassone in collaboration with Dr. R. Dave have created a method to characterize and predict what surfactants and polymers are better stabilizers for a given drug molecule as well as the eventual morphology of the crystal. Furthermore, the combination of surfactants and bio-polymers has also been shown to effectively stabilize drug particles; however, this must be done computationally due to the unfeasible number of experiments necessary to screen behavior for individual classes of drugs.
This work was focused on developing a knowledge base for optimal stabilization of drug nanoparticle suspension systems using experiments and Molecular Dynamics simulations. The stability of a given drug was studied by comparing the relative attachment energies and the corresponding drug crystal entropies for different cleavage crystal faces under the interaction of different polymers and surfactant-polymer mixtures. The model drug, Griseofulvin, a poorly water soluble compound, was combined with various surfactants such as Tween 80 and Sodium Dodecyl Sulfate in combination with bio-polymers Hydroxypropylmethyl Cellulose and Pullulan. It was found that a synergistic effect exists that enhances the stability of the drug when surfactants and polymers are used in combination. For example, in the Griseofulvin system, it was found a mixture of HPMC with anionic surfactant was a better stabilizer than a combination of HPMC with the nonionic surfactant Tween 80. Furthermore, HPMC alone yielded the most dramatic results when compared to any of the other surfactant combinations. All simulation results are consistent with the experimental findings at NJIT in Dr. R. Dave’s group. These models serve as a general guidance to screen and select potentially better stabilizers for poorly water soluble drugs, selected among a large number of stabilizer candidates without testing them all. In addition, this simulation tool can be extended to other API molecules, thus, representing a great benefit for the pharmaceutical industry.
Address Goals
Discovery: The creation and stabilization of pharmaceutical nanoparticles is a complex challenge that incorporates important aspects of pharmaceutical engineering, organic chemistry, and colloidal physics. This project, utilizing both novel experimental methods for the production of organic nanoparticles in combination with cutting-edge computing for subsequent nanoparticle stabilization represents the culmination of many areas of science for the eventual goal of improving our modern pharmaceuticals. The first project utilizing, an emulsion templeted synthesis of pharmaceutical nanoparticles, represents a truly novel technique for the development of nanopharmaceuticals. While in its relative infancy, this procedure will have wide-ranging impacts on the future synthesis of today’s modern drugs. As the pool of available drugs increases, the issue of poor water solubility will need to be addressed by effective, scalable, and most importantly safe techniques to aid in the bioavailability. In addition, the ability to keep nanopharmaceuticals stable and free from agglomeration will take on an even bigger role as nanopharmaceutical processes get more advanced, and demand for more nanopharmaceuticals increases. Computational techniques, such as the Molecular Dynamics simulations described here represent the latest technology for screening stabilizers without ever stepping foot in a laboratory. These techniques will further increase in effectiveness as computers get faster and more complex. The most significant breakthrough for both of these techniques is the emphasis on limiting environmental impacts by using computing in place of experimentation and the utilization of food-grade chemicals. Both of these projects will have a lasting effect on how the future of the pharmaceutical industry morphs from trial-and-error to a more fundamentally sound science.
Learning: Manufacturing homogeneously sized drug nanoparticles involves the mastering of several experimental techniques as well as fundamental concepts in different disciplines in science and engineering including fluid mechanics, colloidal physics, chemistry and pharmacy. This project has provided an excellent experimental and theoretical training for the two graduate students working on the project: Frank Romanski and Eric Jayjock. Both graduate students have successfully designed, constructed, and operated a multi-functional high pressure homogenizer system. The students have been able formulate many novel nanoparticle designs while working in a multi-disciplinary field with students and professors in other universities and of different backgrounds. Moreover, this work has also benefited from Rutgers’ educational infrastructure. Rutgers has a powerful and rapidly growing educational effort in Pharmaceutical Engineering which incorporates a large number of undergraduate students every year in the laboratories. Two undergraduate students in particular, Daniel Fritz and Roger Saez, have been thoroughly trained in these experimental and analytical techniques. One of them, Roger Saez, won the first place in a poster competition for the Society of Hispanic Professional Engineers (SHPE), in Phoenix, Arizona.
As part of reaching the goals of the educational impact, Prof. M. S. Tomassone has developed a new course in Molecular Simulations in Nano-pharmaceuticals ( 16:155:479) providing an immediate, natural avenue for incorporating results from this project into student education. A simple, and effective manner to use results from this project to favorably impact learning has been to use the programs developed in this project as training tools for students who have taken Dr. Tomassone’s course.