Highlight
Novel discoveries in the field of binary superlattice self-assembly
Achievement/Results
IGERT trainee Brian Goodfellow and graduate student Danielle Smith working under the supervision of IGERT faculty member Dr. Brian Korgel at the University of Texas at Austin have made novel discoveries in the field of binary superlattice self-assembly.
Nanocrystals will self-assemble into higher order structures by entropically driven processes. It may seem counterintuitive that an ordered crystal has higher entropy than the disordered colloidal nanocrystal solution from which it grows. However, the superlattice is actually locally disordered, as the nanocrystals have more freedom to move locally within the cages created by their neighbors than they do in a metastable fluid. This phenomenon was utilized to self-assemble binary nanocrystal superlattices. A colloidal solution of 11.5 nm Fe2O3 and 6.1 nm Au nanocrystals in toluene was evaporated on a tilted substrate in the presence of excess oleic acid. The presence of a tilted substrate was critical for the formation of an evaporative front for the deposition of the superlattice at the air-liquid-substrate interface. Excess oleic acid was found to also be necessary to enhance the mobility of the nanocrystals in solution as well as to provide an additional driving force for the superlattice formation through a depletion attraction mechanism.
AB2 superlattices were observed using transmission and scanning electron microscopy (TEM and SEM). The superlattice matched to a simple hexagonal space group number 191. Three different lattice planes were observed in TEM and SEM, including the (001), (100), and (110) planes. See Fig. 1.
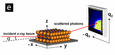
The superlattices were also characterized using grazing-incidence small angle X-ray scattering, or GISAXS. GISAXS was performed in collaboration with Dr. Detlef Smilgies at the Cornell High Energy Synchrotron Source in Ithaca, NY. In GISAXS, an X-ray beam comes in at a grazing angle, scatters off of the sample, and is collected on a 2D detector. The collected diffraction pattern can then be indexed. See Figs. 2-3.
The GISAXS diffraction pattern revealed that there was uniaxial shrinkage of the superlattice in the direction normal to the substrate, which had not previously been reported for binary nanocrystal superlattices. This type of contraction normal to the substrate has been observed in other evaporated films, such as in ordered block copolymers, mesoporous metal oxides, and gold nanocrystal superlattices. The lattice contraction results from the evaporation of residual solvent retained by the capping ligands just after superlattice formation. As the residual solvent evaporates, the superlattice shrinks. But the nanocrystals cannot move laterally with respect to the substrate because their positions are fixed by adhesion to the substrate and as a result, the lattice decreases its total volume with a uniaxial compression toward the substrate.
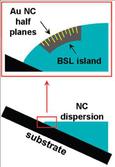
The observation of gold nanocrystal half-plane defects in binary nanocrystal superlattices was also observed for the first time. The insertion of the half-planes is believed to arising from the strain imposed on the superlattices at the curved air/solvent interface. The dislocations relieve this strain as the superlattice forms. See Figs. 4-5.
Address Goals
These seminal developments enhanced the fundamental understanding of nanocrystal self-assembly and could potentially be useful in applications requiring nanocrystal films, such as nanocrystal based photovoltaics.
Further work is being done to observe the dynamics of binary nanocrystal superlattice formation. By understanding the mechanisms of nanocystal superlattice formation, one may be able to tailor a nanocrystal assembly for a specific application. See Fig. 6.