Highlight
Hot News on a Cold Sea: CO2 Levels in Labrador Sea Water
Achievement/Results
Description of IGERT Fellow Francesca Terenzi’s Research that is the Highlight:
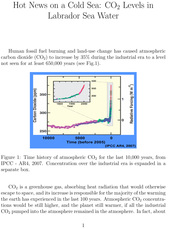
Human fossil fuel burning and land-use change has caused atmospheric carbon dioxide (CO2) to increase by 35% during the industrial era to a level not seen for at least 650,000 years (see Fig.1).
CO2 is a greenhouse gas, absorbing heat radiation that would otherwise escape to space, and its increase is responsible for the majority of the warming the earth has experienced in the last 100 years. Atmospheric CO2 concentrations would be still higher, and the planet still warmer, if all the industrial CO2 pumped into the atmosphere remained in the atmosphere. In fact, about 1 half of that CO2 has left the atmosphere, the majority entering the oceans.
Better quantifying the oceans role in the perturbed carbon cycle and how this role might change is an important component of climate-change research and is the topic of study by a team at the NASA Goddard Institute for Space Studies (GISS) led by Dr. Timothy Hall
CO2 enters the ocean by gas exchange at a rate determined by the atmosphere-ocean concentration difference. Once in the ocean it evolves chemically, due to both inorganic and biological processes, and is transported by ocean currents and turbulence away from the surface. The complexity of these processes, and the fact that the preindustrial ocean already had a high concentration of dissolved carbon, makes estimation of industrial carbon in the ocean much harder than the atmosphere.
It turns out that these difficulties can be overcome by making a powerful approximation. While CO2 in the atmosphere can stimulate additional photosynthesis, ocean phytoplankton is in large part limited by the availability of various nutrients and cannot incorporate the additional carbon in new biomass. To the extent this is true, the elevated dissolved carbon in the ocean due to human activity is inert, carried passively by ocean currents and turbulence. This greatly simplifies the problem of its estimation, rendering it amenable to techniques of tracer transport analysis.
Address Goals
Francesca Terenzi, a doctoral student and IGERT fellow in the GISS team, and her coauthors, have recently developed and applied novel tracer-analysis techniques to the estimation of CO2 uptake by the Labrador Sea in the North Atlantic Ocean (Terenzi et al., 2007, 1). The North Atlantic is responsible for a disproportionately large share of ocean carbon uptake, and the Labrador Sea, due to its active vertical mixing, is one of the few rapid conduits from the surface to the deep ocean (see Fig.2). They found that the carbon concentration of the Labrador Sea is far below the level that would be at equilibrium with the atmosphere (Fig.3). Terenzi et al. 1 also conclude that the preindustrial Labrador Sea may well have been a weak source of carbon to the atmosphere, in contrast to the conventional view (Fig.4).
In order to estimate Labrador-Sea uptake, a version of the transit-time distribution (TTD) inference technique was used, which had been previously developed by the ocean-carbon team at GISS. This method avoids the commonly made assumptions that ocean mixing is weak and that carbon in surface waters faithfully track atmospheric CO2 trends (e.g., Sabine et al., 2004, 2). Moreover, the TTD technique can be used to estimate the history of the industrial carbon uptake, rather than just a snapshot in time. Essentially, the method exploits measurements of other inert tracers with no natural background to estimate ocean transport due to both bulk advection (large bodies of water moving en masse) and turbulent diffusive mixing. These estimates are then used to propagate industrial carbon in surface waters into the interior ocean. The surface-water carbon is determined by enforcing consistency between the air-sea flux and the rate of increase of carbon mass in the Labrador Sea volume.
Terenzi et al. 1 find that the Labrador Sea cannot keep pace with increasing atmospheric CO2. In essence, the small surface area of the Labrador Sea acts as a bottleneck, limiting the access to its large interior volume. Combining current-day measurements of total Labrador-Sea carbon (industrial plus natural) with their estimates of total industrial carbon uptake, Terenzi et al. 1 also estimate the preindustrial carbon concentrations in the Labrador Sea. In contrast to the conventional view, they conclude that in preindustrial times the Labrador Sea most likely had a higher concentration of carbon than the atmosphere, and was thus a source to the atmosphere. Sometime in the mid 20th century the relative concentrations switched signs, and the Labrador Sea became a sink.
Currently, Terenzi is exploring further aspects of the analysis. For example, in Terenzi et al. 1 it was assumed that rates of Labrador Sea currents and mixing are constant in time, when, in fact, they are known to be episodic. Terenzi 3 is developing and applying simple models of time-varying transport, and one of the aims is to apply them to tracer data and estimate the influence of such variability on carbon uptake.
This project was successful in that it combined the expertise of Dr.Hall, and Dr. Khatiwala, respectively a physicist and a theoretical oceanographer with extensive research in transport dynamics in the atmosphere and in the ocean, with Dr. Rodehacke, and Dr. LeBel, which are physical oceanographer experts.
F. Terenzi was funded as a Fellow of a National Science Foundation (NSF) Integrative Graduate Education and Reserach Traineeship (IGERT) in Applied Mathematics and the Earth & Environmental Sciences, DGE-0221041, at Columbia University’s (CU) Department of Applied Physics & Applied Mathematics (APAM). Dr. Timothy Hall is a Senior Scientist at NASA GISS, and Adjunct Professor in APAM at CU. Dr. C. Rodehacke is now a Research Scientist at Alfred Wegener Institute for Polar and Marine Research in Hamburgh, Germany. Dr. S. Khatiwala and Dr. D. LeBel are Research Scientists at the Division of Ocean and Climate Physics at Lamont Doherty Earth Observatory, CU. This work was further funded as its own project under a NSF Chemical Oceanography Program, OCE – 06 – 23366.